Moderna continues its string of partnerships / M&A in the gene therapy space: now its Generation bio
With their mountains of cash, Moderna is fueling their investments into Cell & Gene, now signing a pact with Generation Bio for its novel delivery systems. Under the agreement, Moderna will have an option to license Generation Bio's cell-targeted lipid nanoparticle (ctLNP) and closed-ended DNA (ceDNA) technology for two immune cell programs and two liver programs, with an added option for a third immune cell or liver program.
Generation Bio will receive $40M cash upfront and $36M equity investment issued at a premium over recent share price from Moderna, and potential future development, regulatory and commercial milestone payments, and royalties.
Its an interesting move from Moderna, who have now made a series of pacts / partnerships in the past year, cementing their resolve for this space
In September 2021, Moderna formed a pact with Vertex for gene editing CF programs [source]
In November 2021, Moderna signed a pact with Metagenomi for gene editing technologies [source]
In October 2022, Moderna signed a deal with Autolus for proprietary binders [source]
In January 2023, Moderna aquired Oriciro genomics for cell-free synthesis and amplification of plasmid DNA [source]
In February 2023: Partnership with Life Edit Therapeutics with the aim of developing next-gen gene editing therapies for hard-to-treat diseases [source]
Source: Reuters
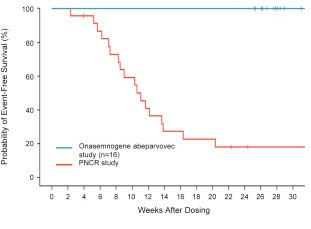
Novartis shares Zolgensma long-term data demonstrating sustained durability up to 7.5 years post-dosing
Zolgensma is really amazing… the GOAT 🐐 (Greatest Of All Time)
In its latest data release, Novartis shared data of patients up to 7.5 years post dosing demonstrating maintainment or achievement of all assessed motor milestones, including independent walking (!!!) in all children (i.e., 100%)
“I have had the privilege of observing some of the children included in the LTFU studies since they started their Zolgensma clinical trial journey, and the fact that we’re seeing them maintain and, in some cases, gain motor milestones when they are nearly eight years old is truly transformational” Dr. Jerry R. Mendell of Nationwide Children’s Hospital
To put the power of this medicine into perspective, its important to understand that without treatment, most children with type 1 SMA (the most severe form) die before their second birthday. The Kaplan Meier below (from the START trial) hopefully illustrates this
Source: endpointnews; SMA background info; Kaplan Meier curve
Quick take news
Athenex discloses patient death, clinical hold on CAR-NKT trial as it seeks strategic alternatives [link]
Gracell has entered an unusual, non-exclusive global deal with Seagen (right before the Pfizer M&A) to conduct pre-clinical research on Seagen’s cell therapy products and acquire non-exclusive rights to five of Seagen’s cell therapies [link]… details of the deal are still a mystery for now …
Aurion Biotech Receives Approval from Japan’s PMDA for New Drug Application for its allogeneic cell therapy to treat corneal endothelial disease [link]
What we liked in the social media space
Linkedin Post from Neil Grubert on the greater acceptance of biomarkers in the gene therapy space —> Link
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer