Caught My Eye
New data release for NTLA-2002 in HAE released by Intellia
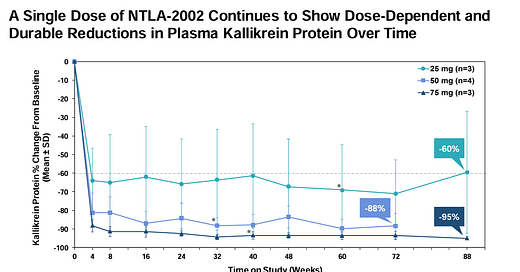
Intellia Therapeutics released long-term data from its Ph I/II study of NTLA-2002, an in vivo CRISPR-based gene editing therapy for hereditary angioedema (HAE). The HAE patients enrolled had a minimum of three attacks from the disease in the three months prior. At an average 20 month follow up period, 8/10 patients were attack free, corresponding to a 98% reduction in mean monthly HAE attack rate. The most common TEAEs were mild infusion-related reactions in 7/10 patients - no serious adverse events were reported. Importantly, 100% of patients were able to discontinue prophylaxis treatment after NTLA-2002 dosing and remain free of chronic prophylaxis treatment. See the data for yourself below 👏
Source: IntelliaIR
Caribou Bio retrospective analysis suggest HLA matching may help persistence of allo-CAR-Ts in LBCL
I’ll start by caveating that the analysis was retrospective, and thus of course subject to research bias: however, the concept makes sence, and the very early data suggest an incremental benefit vs previous allo-CAR-T data.
As a reminder, the key issue with allo-CAR-Ts is duration of response: whats neat here, is that the HLA matching approach seems to improve on this key limitation: data from n= 46 patients suggests mPFS of 14.4 mths when 4 ≥ HLA matches, vs 2.9 mths in the <2 HLA matches. Moving forward, Caribou plans to enroll ~20 more patients with 4 ≥ HLA matches to confirm findings, with a potential pivotal trial starting in H2’25.
Regarding addressable patient population, Caribou has ~13 different batches of CD-010 at hand, which it estimates should cover ~90% of patients in 2L LBCL. Could this be the start of a new (revival) trend for allo cell therapies ? Tbd whether they will ever manage to each auto CAR-T levels, but early data does seems promising !
Source: Caribou IR
Quick take news
Cellectis Receives Orphan Drug Designation for UCART22, its [globenewswire.com]
Adicet Bio’s ADI-001 Gains FDA Fast Track for Lupus Nephritis [Adicetbio.com]
Bilateral gene therapy in children with autosomal recessive deafness 9: single-arm trial results [Nature Medicine]
Quell Therapeutics Advances QEL-001, its Multi-modular Engineered CAR-Treg Cell Therapy, into Efficacy Cohort of LIBERATE Phase 1/2 Trial in Liver Transplant Patients [Yahoo]
New data published for satri-cel, a CLDN18.2 CAR-T in GI cancers: ORR of 38.8%, mPFS of 4.4 mths and mOS of 8.8 mths [Nature Medicine]
uniQure announced that the FDA has granted RMAT designation for its investigational gene therapy AMT-130 for the treatment of Huntington’s disease [morningstar]
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer