A belated happy 4th July Independence Day to all the U.S. folks ! Not much in the Cell & Gene world, so keeping it short this week :) Enjoy the weekend ahead !
What I read this week
» J&J and Legend announce positive readout from their Ph III CARTITUDE-4 study of Carvykti in 2L MM. The study was evaluating Carvykti vs standard of care (PVd or DPd) in 2L+ r/r MM and whilst they did not provide specific data points, they noted Carvykti treatment led to a “statistically significant and clinically meaningful improvement” in overall survival 👏… Carvykti continues to have spectacular data - now even more differentiated vs BMS Abecma, which is approved in 3L MM, but without survival data — Source: BioSpace; FiercePharma
» First hemophilia patients treated with Hemgenix in Europe. This comes >1 year after the EC approval for Hemgenix (Feb’23) —> yet another demonstration of the challenges with commercializing Gene therapies in the old continent: i.e., generally ridddled with access delays and / or non-reimbursement.
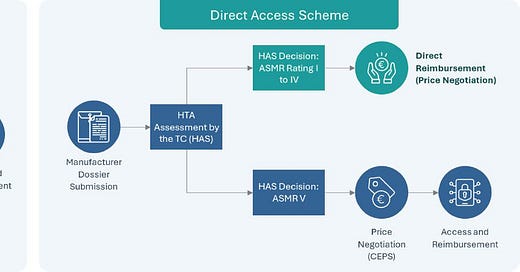
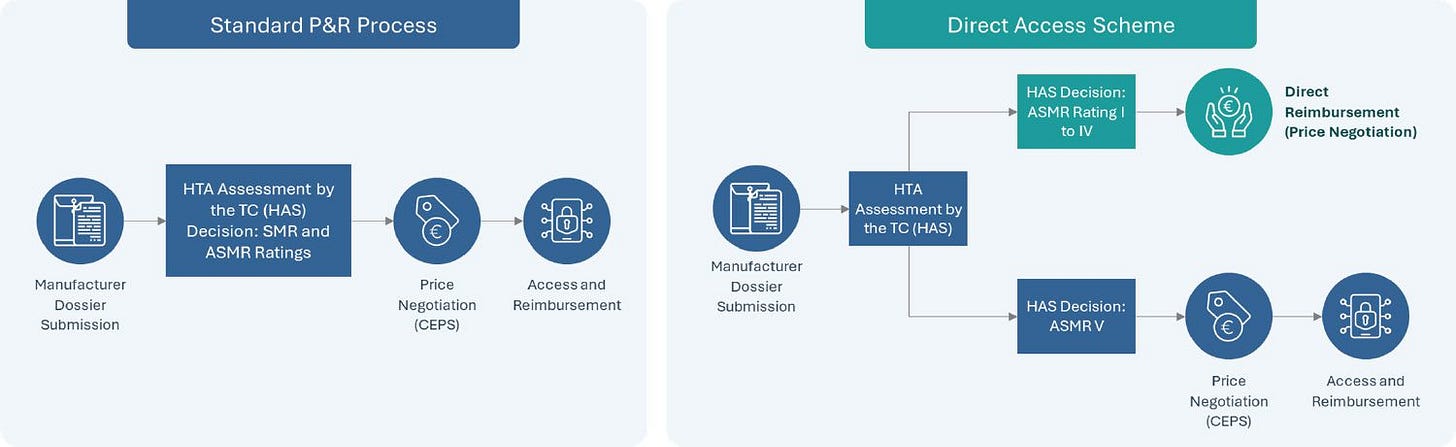
The fist two patients to receive treatment were based in France, and got access via the new Direct Access scheme, which enables drugs with demonstrated benefits (i.e., ASMR I-IV) to get access whilst price negotiations are ongoing. For the market access aficionados out there, it will be interesting to see how the Hemgenix negotiations with the CEPS pan out: will certainly serve as an important case study for future gene therapy manufacturers wishing to pursue access through this route ! A visual below of the new Direct access scheme for those interested, courtesy of TLS
Of note that UK patients have also recently received access to Hemgenix under a managed entry acccess (MEA) scheme; under the UK MEA, data will be collected over five years to enable both the long-term effectiveness, and any adverse liver toxicity caused by the transgene, to be monitored.
In the meantime, Uniqure (who developed Hemgenix) have sold the commercial viral vector manufacturing facility in Lexington, Massachusetts, to Genezen —> Genzen will therefore now supply Hemgenix and uniQure’s clinical portfolio from this site —> a notable move from Unique to reduce expenses and to pay off debt.
Sources: worldpharmaceuticals.net; T-LS; fiercepharma
Quick take news
🥺 FDA rejects Rocket Pharmaceuticals’ gene therapy for ultra-rare condition, another setback for field [source] Looks like a CMC issue, which should in principle be addressable relatively quickly … [source]
Chroma Medicine Announces Exclusive License Agreement with the Whitehead Institute for Novel Epigenetic Editing Technology [source]
Rejuvenate Bio received a $4M grant from the California Institute for Regenerative Medicine (CIRM) to advance RJB-0402, a gene therapy for desmoplakin gene variant arrhythmogenic cardiomyopathy (DSP ACM) [source]
Codexis, Inc. announced an asset purchase agreement with Crosswalk Therapeutics for its investigational gene therapy assets [source]
Interesitng articles
Jacob Plieth covered in oncologypipeline an overview of fast-manufactured CAR-Ts - according to him, a three way race across Gilead, Novartis and BMS, but with a fourth emergent competitor Gracell, now part of AZ. —> Link to the article Below a nice table summmary, available in the link above
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer
Have I missed anything? Is there something you would like to hear more about? let me know !