Cell & Gene Weekly
FDA extends Elevidys label, New data from Taysha in Rett, new paper in Nature Med on access to pediatric C&G therapies, and more...
FDA expands Elevidys label label in DMD
FDA granted Sarepta’s gene therapy, Elevidys, full approval for ambulatory DMD patients >4 yrs and accelerated approval (AA) to non-ambulatory patients, effectively expanding on the former AA of Elevidys in 4-5 yr old ambulatory patients.
The big uncertainty around this approval was centered around the fact that the drug had missed its primary endpoint in a large Phase 3 trial last year, but had met all key secondary endpoints [Oct'23 press release]. Perhaps Pfizer’s recent setback in a similar Ph III setting put the pressure on the FDA to fully approve / expand the scope of Sareptas gene therapy. Certainly a tight / controversial decision, with a narrow outcome at an ad-com ~1 yr ago relating to the original AA for the drug. According to statnews the decision was made by Peter Marks, the agencys director for CBER, overuling several review teams and “top lieutenants” who had cast doubts on the benefit of treatment. Regardless, an important achievement for patients and for the broader cell and gene space as a whole ! Congrats to Sarepta !
Quick take news
» Allogene announced initiation of a pivotal Ph II trial to “leap-frog” their competition. With the fundamental challenges plaging allo gene therapies (vs their autologous counterparts), we’re starting to see these companies trying to carve out space for themselves in new / innovative ways. In the latest unconventional strategy, we see Allogene attempting to move directly into 1st line by leveraging an MRD test to detect patients who are most likely to relapse. They will use Foresight CLARITY MRD test to identify patients and administer a one-time infusion immediately upon detection of MRD at the completion of six cycles of R-CHOP or other standard 1L. All in all, Allogene are betting on changing clinical practice + novel therapy: hard task, but certainly bold — Source: AllogeneIR
» Taysha tx announced new data for their Rett Syndrome gene therapy, TSHA-102. Despite some early signal of efficacy, the data is very preliminary. Indeed, only last week I commented on the relevance of public companies posting data for n=2 patients, noting the risks with such press releases. Seems like investors were not keen on this type of press release last week with Cabaletta (stock trading -25% intraday) and this week with Taysha (-35% intraday). #ouch. After all, lack of a placebo arm, small sample size and in the case of Taysha, the need for much more data given recent approval of Acadia’s Daynue in Rett, opens up to significant uncertainty. — Source: TayshaIR
» Roche partners with Ascidian to develop exon editing drugs. As part of the deal, Ascidian will receive $42 M upfront, and up to $1.8 bn in biobucks + royalties on global commercial sales. For those interested, have a look at Ascidian website: the idea behind their tech is to edit RNA exons with the logic that mutations in the exons, which during the transcription of DNA to RNA are spliced to form mRNA, can result in malformed, disease-driving proteins. — Source: AscidianIR; Fiercebiotech
» Freelance aquires SwanBio and rebrands into Spur Therapeutics. The new 99% Syncona-owned company is set to have a valuation of around $130 M, with Syncona committing $50 M to fund development of the company's pipeline — Source: Syncona
What I read this week
Very interesting new paper published in Nature Medicine entitled “Enhancing pediatric access to cell and gene therapies”.
The paper brings to light some of the challenges associated with pediatric gene therapy development and proposes non-standard approaches to overcoming such challenges. They provide a couple of examples of failures to bring to the market C&G therapies for pediatric indications, namely an ADA-SCID gene therapy from SR-TIGET / Orchard and a CD22-targeting CAR-T from academic investigators that have not reached U.S. market commercially.
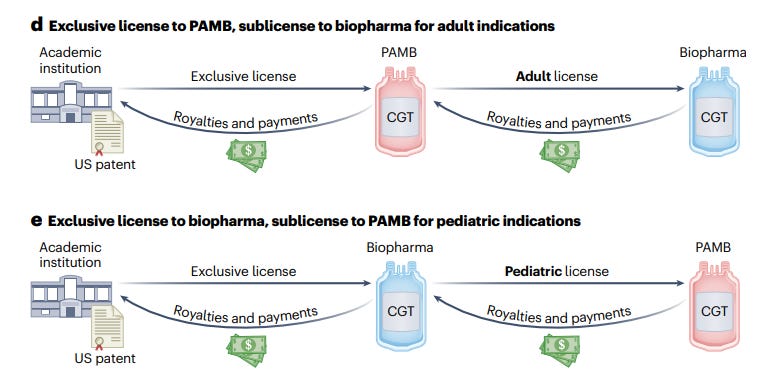
As a first step forward to overcome said challenges, the authors propose creation of a Pediatric Advanced Medicines Biotech (PAMB), an entity that would conduct registrational trials, sponsor biological license applications and commercialize approved CGTs for children. Such PAMB would work closely with the academic ecosystem (pediatric centers of excellence, academic medical centers and research institutes) to identify CGTs ready for late-phase pediatric testing; use academic manufacturing to reduce costs; and achieve regulatory milestones as early as possible to recoup a revenue stream from payors.
Screenshot below for those interested: two interesting models involving academic - PAMB - tradittional biopharma partnership for succesful commercialization of pediatric gene therapies
My favourite inspirational quote from the publication.
“Just as innovation and commitment created these new therapeutic possibilities, so can innovation and commitment create new models for making these therapies accessible to all children in need.”
#Kudos to the authors for such an interesting article and for raising such an important topic 👏
Source: Nature Medicine
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer