It’s always great to finish the week off with an FDA approval for Cell & Gene therapy: yesterday, BMS announced the green light from the FDA for Breyanzi in MCL. 👏Enjoy the read and have a good weekend !
BMS gets FDA nod for Breyanzin in Mantle Cell Lymphoma
This marks the 4th approval in subtypes of NHL, making it the CAR T with the broadest array of B-cell malignancies. The approval is based on TRANSCEND NHL 001, which enrolled r/r MCL (3L+) – in the n = 68 patients evaluated for efficacy, 85.3% responded to treatment, of which 67.6% were CRs. mDOR was 13.3 mo with a median follow-up of 22.2 mo.
Source: businesswire
Adaptimmune partners with Galapagos for solid tumor TCR-T cell therapy program
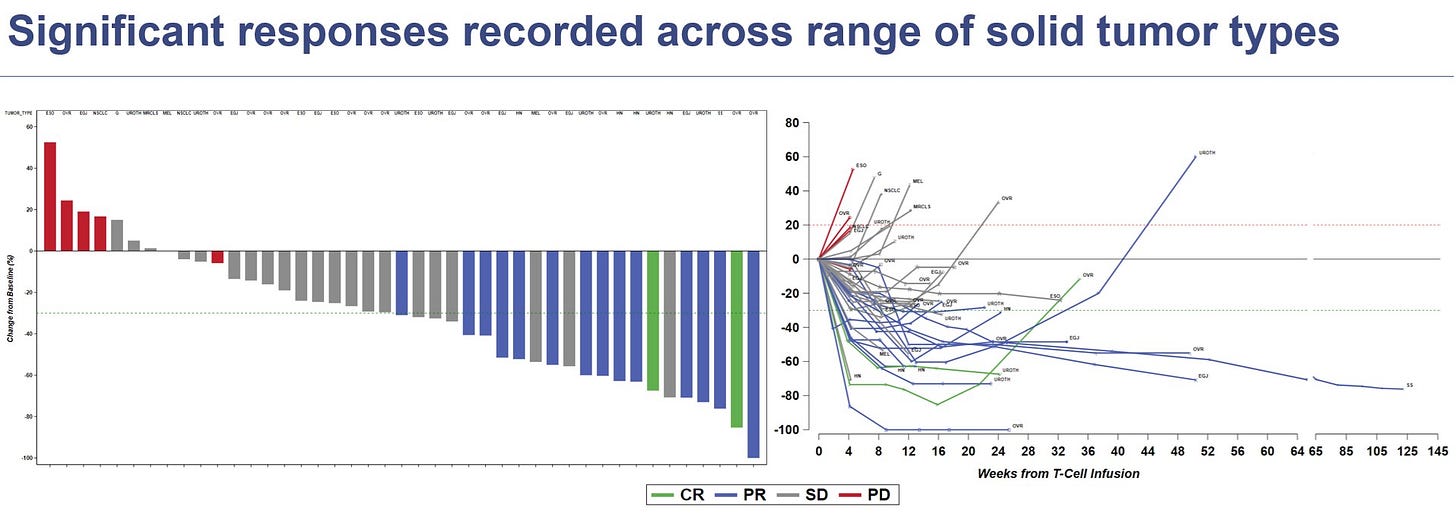
The deal revolves around Uza-cel, a MAGE-A4 TCR T-cell therapy currently in Ph 2 in solid tumor indication, including head & neck and ovarian cancer. Adaptimmune has previously disclosed some pretty good data on the asset, showing PR’s and even a few CR’s for this agent, either as a monotherapy, or in cominnation with IO (nivolumab). The idea behind this collaboration is to develop uza-cel using Galapagos’ decentralized manufacturing platform, based on the fact that in vitro testing of uza-cel on this platform yields early phenotype T-cells that could improve efficacy and durability compared to uza-cel centrally manufactured on Adaptimmune's platform.
As a reminder, in their pooled Ph 1 trial, Adaptimmune had 50% response rate in 26 patients with ovarian, urothelial, and head & neck cancers; the response rate increased to 75% in patients who received three or fewer prior lines of therapy. 👏
As part of the deal, Adaptimmune will receive an upfront payment of $70 M, R&D funding of $30 M, option exercise fee of $100 M, and $465 M in “biobucks”, along with royalties on sales.
Source: Galapagos IR; Adaptimmune IR; Data for Uza-cel
Quick take news
BlueRock Therapeutics receives FDA Regenerative Medicine Advanced Therapy designation for Parkinson's disease cell therapy candidate bemdaneprocel [link to article]
Ultragenyx Pharmaceutical Says Glycogen Storage Disease Treatment Met Endpoints [Link to article]
Chinese Scientists Achieve World's First Diabetes Cure Using Cell Therapy [link to article]
Parkinson’s gene therapy increases brain GCase in nonhuman primates [link to article]
FDA Grants Orphan Drug Designation for CAN-3110 for the Treatment of Recurrent High-Grade Glioma [link to press release]
What I read this week
A biotech reckoning: 'We've lost our luster in cell therapies' [fiercebiotech.com]
Cell and gene therapy companies trip at scalability hurdle - Pharmaceutical Technology [pharmaceutical-technology.com]
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer