Sangamo Tx announces two deals in the span of a week, one with Prevail and the other with Chroma Medicine
After losing two Big Pharma partners Biogen and Novartis in quick succession earlier in the year, Sangamo has established two new deals (… in quick succession…), one with Chroma medicine and the other with Prevail (an Eli Lilly subsidiary)
In the deal with Chroma Medicine, the companies will research and develop epigenetic medicines leveraging zinc finger proteins (ZFPs) for sequence-specific DNA recognition. Sangamo is entitled to an upfront payment, and if Chroma exercises its option to license any or all targets, an additional option exercise payment.
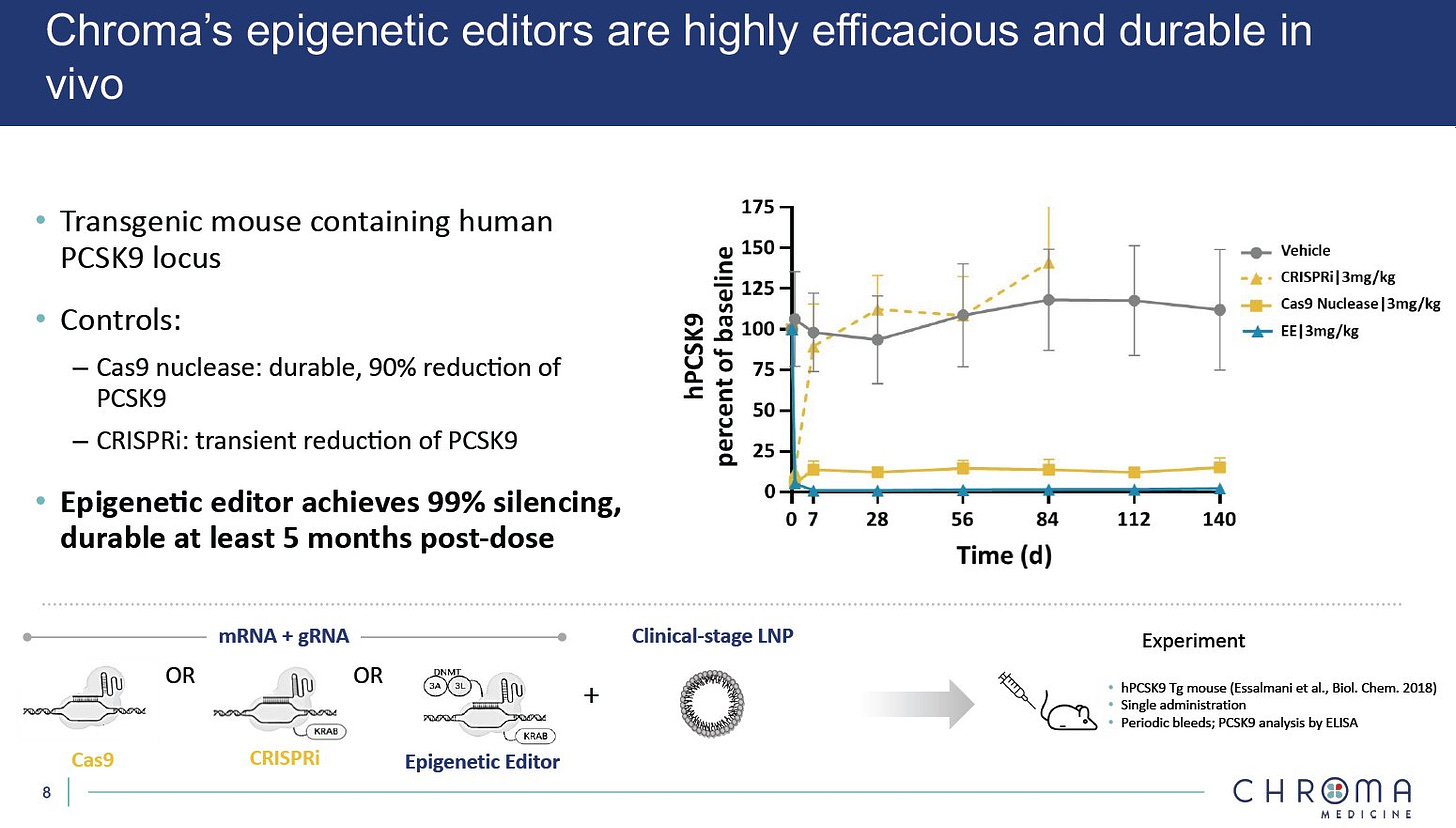
Chroma so far has demonstrated some impressive preclinical data with PCSK9, with 99% silencing and durability of at least 5 months, in mice. Certainly impressive data vs some of the other PCSK9 in RNAi or in vivo gene editing approaches. Will be interesting to see in which direction the two companies take the epigenetic collaboration
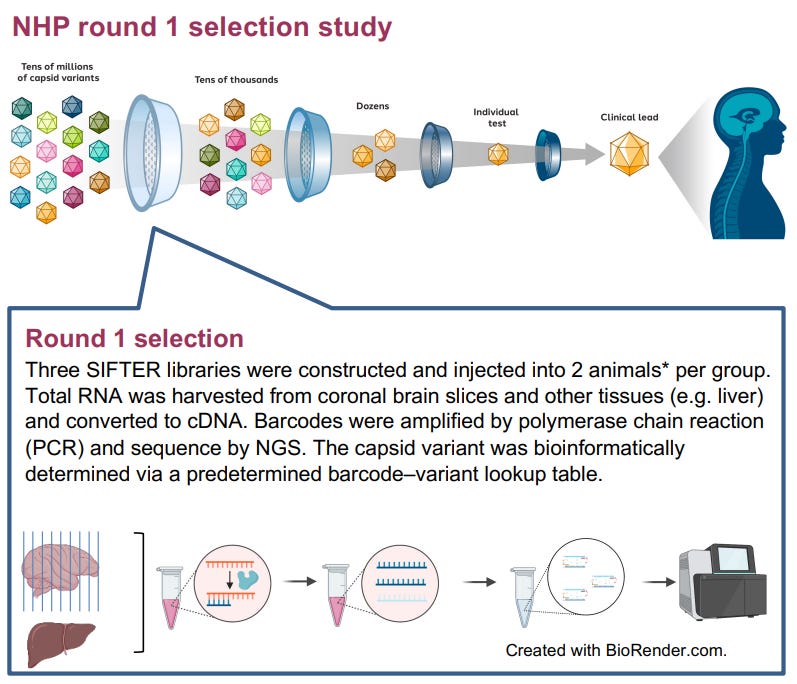
In the deal with Prevail Tx, the companies will pursue proprietary AAV capsids for certain neurological targets. Under the terms Sangamo could receive developmental milestones of up to $415 M and commercial payments of up to $775 M, plus tiered royalties. The capsids in question are produced through the SIFTER platform (Selecting In vivo For Transduction and Expression of RNA), and have demonstrated a potential for high efficiency delivery of investigatory gene therapy constructs to the central nervous system in pre-clinical animal models
Source: Fiercebiotech, business wire, Sangamo website
Neurogene and Neoleukin merge in a move to fund operations until 2H 2026
Neurogene and Neoleukin Tx announced a merger agreement to combine the companies, with the new company expected to operate under the name “Neurogene Inc”. In addition to the merger, Neurogene also raised $95 M private financing led by new and existing institutional investors. Neurogene expects to exit the transactions with around $200 M to its name.
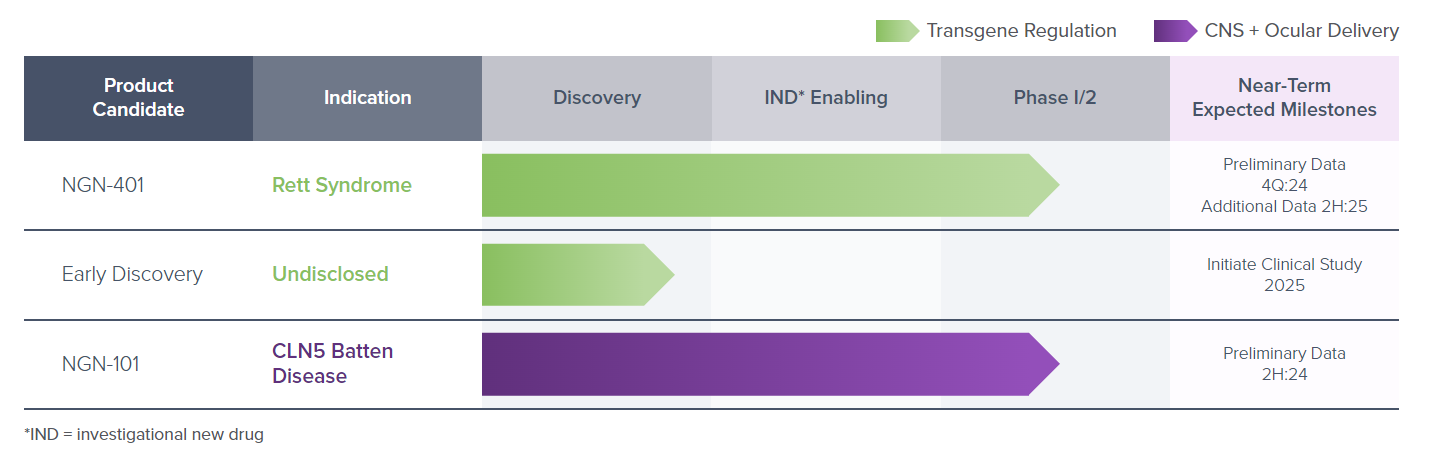
The company has trial clinical stage assets for Rett syndrome and CLN5 Batten disease with preliminary data from Ph 1/2 clinicals trials due in the second half of next year.
Source: Fiercebiotech
Status check on commercialization of Cell and Gene therapies
Novartis, J&J, reported earnings this week, so here is the initial assessment of how Cell and Gene therapies are doing in the marketplace:
Novartis [source]
Zolgensma QoQ was unchanged at $311 m —> Novartis has likely reached the plateau until the IT formulation comes in
Kymriah QoQ declined from $135 m in Q1 ‘23 to $129 m in Q2 ‘23—> As per previous quarters we seem to be observing a slow decline, likely driven by the other competitor CD19 CAR-Ts moving into earlier line settings…
J&J / Legend [source]
Carvykti net revenue increased from $72 m to $117 m in Q2 2023 —> with gradually increasing internal manufacturing capacity, the commercial agreement with Novartis for clinical trial supply of Carvykti [link] and the stellar data, it looks like there is a proper ramp up ongoing
Quick take news:
Nkarta Updates Clinical Progress of CAR-NK Cell Therapy NKX101 for Patients with Relapsed or Refractory Acute Myeloid Leukemia. In patients with r/r AML treated with a three-dose regimen of NKX101 at 1.5 billion cells per dose after fludarabine/Ara-C for lymphodepletion, n=6 [source]
4 of 6 patients achieved complete response (67% CR/CRi, 50% CR rate)
2 CRs with MRD negativity
1 patient deepened response to MRD negative CRi with additional cycles
Korro Bio and Frequency Therapeutics Announced Merger Agreement to create a Nasdaq-listed genetic medicines company focused on advancing Korro Bio’s wholly owned portfolio of RNA editing programs [source]
Sanofi expanded its existing collaboration with Scribe: Sanofi will receive exclusive license to use Scribe’s CRISPR X-Editing (XE) genome editing technologies for the development of in vivo therapies, including sickle cell disease [source]
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer
Have I missed anything? Is there something you would like to hear more about? let me know !