Cell & Gene Weekly
Re-dosing gene therapies, preclinical data for a GLP-1 gene therapy, cell therapy for diabetes, and more...
Happy Friday, enjoy the read and wishing all a great weekend ahead !
What I read this week
» New data from Intellia suggests re-dosing of gene therapies is possible: the findings came in the context of a group of patients who had received low doses of a gene therapy that did not reach therapeutic levels during a Ph I dose-escalation study in ATTR amyloidosis. Fast forward » (re-)administering a second, higher dose, resulted in a significant reduction in serum TTR levels, i.e., 90% median reduction. While redosing isn't currently planned for this program, it opens up for new approaches in other therapeutic areas where patients might need multiple doses to reach desired therapeutic effects... and for those of you thinking that redosing defeats the whole purpose of gene therapies, i’d argue that, no, the value of gene therapies is not only the one-and-done element of it ! - Source - FierceBiotech
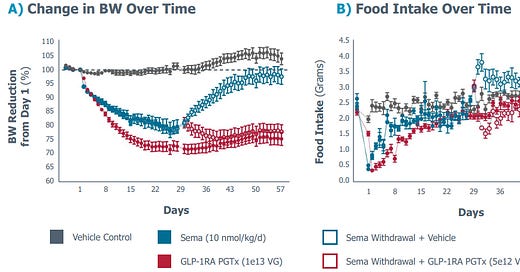
» New preclinical (mouse) data from Fractyl's GLP-1 gene therapy suggests maintenance of weight loss post use of GLP-1 agonist treatment. In most recent data release, mice in the crossover arm that were treated and then stopped semaglutide to receive the GLP-1 gene therapy, maintained a 17% fat loss / 22% total weight loss; on the contrary, the ones that did not receive the GLP-1 gene therapy regained all but 1% of their baseline weight. Considering the high discontinuation rates of GLP-1s in the real world context, this could be an interesting “one-time” approach to maintain benefits of the GLP-1s. Below body weight change over time from the study - Source: FierceBiotech; Fractyl
» Vertex Pharmaceuticals announced positive results from their Ph 1/2 trial of VX-880, a stem cell-derived islet cell therapy for T1D. All patients (n=12) showed islet cell engraftment and glucose-responsive insulin production by day 90, achieving target HbA1c levels and over 70% time-in-range. 11 reduced or eliminated insulin use, and 3 patients with at least 12 months follow-up met primary endpoints of eliminating severe hypoglycemic events and achieving insulin independence. - Source: VertexIR
» Novo Nordisk purchases 2seventy bio’s hemophilia A program + rights to the megaTAL tech (outside of oncology), for up to $40 M. This comes five months after the divesture of most of 2Seventy’s pipeline + R&D staff to Regeneron, and builds off an earlier pact inked back with Novo Nordisk in 2019: a clear strategic shift / focus on Abecma commercialization in the U.S. - Source: FierceBiotech
Quick take news
NHS England has secured a commercial agreement with CSL Behring B.V. to make a Hemgenix, a gene therapy for Haemophilia B, available to NHS patients via the Innovative Medicines Fund. [source]
Beam Therapeutics Announces First Patient Dosed in the Phase 1/2 Study of BEAM-302 in Alpha-1 Antitrypsin Deficiency (AATD) [source]
Kyverna's KYV-101 Receives U.S. FDA IND Clearance for Treatment of Patients With Treatment-Refractory Stiff-Person Syndrome in the KYSA-8 Phase 2 Trial [source]
*Any views and opinions expressed herein are those of the author (Marco Sabatini) and do not necessarily reflect those of his employer
Have I missed anything? Is there something you would like to hear more about? let me know !